
Introduction. New guidelines for the management of dyslipidemia and lipid modification in order to reduce the risk of cardiovascular (CV) events have been recently published by the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). New recommendations regarding the target value of plasma lipids in very high and high CV risk patients have been provided, in addition to an estimate of the CV risk with a new Systematic Coronary Risk Evaluation (SCORE) chart. Few data have been reported on the management of dyslipidemia in chronic myeloid leukemia (CML) patients treated with nilotinib, and the association with arterial occlusive events (AOEs). We therefore analyzed a large real-life cohort of Italian patients with CML treated with nilotinib outside of clinical trials and evaluated the association between AOEs and plasma lipoproteins levels; moreover, we estimated the prognostic value of the new SCORE chart to predict AOEs. The secondary endpoint was to report the management of dyslipidemia in the clinical practice.
Methods. We identified 233 adult patients with CML who were treated in 20 Italian centers with nilotinib. All patients were stratified into low to moderate (SCORE ≤ 5%) or high to very high (SCORE risk >5%) CV risk, according to the new version of the SCORE 2019. We recorded concentration levels of cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerides at diagnosis of CML, before starting ponatinib and therefore after 3, 6 and 12 months of treatment. All AOEs (cerebrovascular, peripheral vascular and CV events excluding hypertension) were considered.
Results. The median age was 50 years (range 20-88) and the Sokal score was intermediate-high in 45.5% of patients. The median follow-up was 5 years (range 3.4-10.5). Nilotinib was administered as first line of therapy in (72%) of cases or second or subsequent lines of treatment for inefficacy (20.9%) or intolerance (7.1%). At baseline, nilotinib was administered at the following doses: 800 mg/day in 9.3% of patients, 600 mg/day in 87% of patients, 400 mg/day in 3.1% of patients and 300mg in 0.6% of patients, respectively. The median time of drug exposure was 60 months (range 2-155). The 48-month cumulative incidence rate of AOEs was 14.1±2.7%.
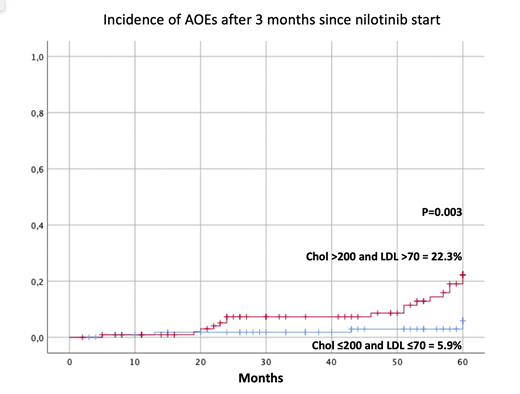
Patients with cholesterol plasma levels > 200 mg/dL and LDL >70 mg/dL at baseline and 3 months after starting nilotinib, showed a significantly higher incidence of AOEs (24.5±7.3% vs 11±2.7%, P=0.02 and 22.3±4.9% vs 5.9±2.6, P=0.003, respectively) Figure 1. Patients with triglycerides levels > 200 mg/dL 3 months after starting nilotinib, showed a significantly higher incidence of AOEs (56±20.5% vs 13.3±2.7%, P=0.011)
Patients belonging to the high and very high SCORE risk group showed a significant increase of AOEs (32.8.1±9% vs. 9±1%±2.6%, p=0.001). In multivariate analysis, statistical significance of cholesterol plasma levels > 200 mg/dL and LDL >70 mg/dL after 3 months and high-very-high SCORE was maintained (P=0.018, HR=3.4, 95% CI=1.2-9.4 and P=0.004, HR=3.5, 95% CI=1.5-8.2, respectively). Overall, 46 patients (20.5%) presented dyslipidemia at CML diagnosis and 65 (29%) at the start of treatment with nilotinib. Despite dyslipidemia, only 6 patients were taking statins during the treatment with nilotinib and only 5 started it after 3 months of nilotinib: 3 patients were treated with rosuvastatin and 2 with pravastatin.
Conclusions. Our findings suggest that a proper control of dyslipidemia, keeping cholesterol and triglycerides plasma levels ≤ 200 mg/dL and LDL ≤70 mg/dL is associated with reduced risk of AOEs in CML patients treated with nilotinib. An under estimation of the clinical importance of elevated plasma lipids as a risk factor for AOEs events represents a possible issue in the real-life.
Abruzzese:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bms: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Galimberti:Incyte: Honoraria; Novartis: Speakers Bureau. Castagnetti:Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Pregno:Incyte-Italy,: Membership on an entity's Board of Directors or advisory committees, Other: conference reports; Novartis-Italy: Membership on an entity's Board of Directors or advisory committees, Other: conference reports; Pfizer-Italy: Membership on an entity's Board of Directors or advisory committees, Other: conference reports. Bocchia:CELGENE: Honoraria; Incyte: Honoraria. Gugliotta:Novartis: Honoraria; Incyte: Honoraria; Pfizer: Honoraria. Foà:Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal